We probably all know someone who suffers from dementia, from grandparents, acquaintances, or maybe even friends. As dementia is getting more prevalent, especially with increasing age, a cure is becoming more and more important in society. Nowadays, 1 in 5(!) people in the Netherlands will be diagnosed with a form of dementia, which affects more than 300.000 people per year. For women, chances of being diagnosed with dementia at older age can go up to 1 in 3 women. Not great statistics for #girlpower.
Dementia is an umbrella term for progressive neurodegeneration and cognitive impairment, or where brain tissue breaks down over time. This negatively impacts daily functioning and can tremendously influence one’s life. Generally speaking, the most common forms of dementia are Alzheimer’s disease (AD), frontotemporal dementia (FTD), dementia with Lewy bodies (DLB), and vascular dementia (VaD). Each of these types of dementia has its own subtypes, with accompanying complex symptoms. Of these four main types, AD is the most common form of dementia (~70%), and also the most widely known in society.
As most types of dementia have certain clinical symptoms, some also have distinct genetic hallmarks and even biomarkers that help us in the diagnostic process. Biomarkers are indicators (often proteins) of a biological process or disease, which can be measured in, for example, blood, urine, or cerebrospinal fluid (CSF) in the case of neurological disease. Some proteins can act as indicators of AD, such as amyloid beta (Aβ) or phosphorylated tau (pTau) in the CSF and blood. These proteins will be further explained in the next paragraph. Together with a clinical diagnosis and MRI, this can be an indication of a disease, in this case of AD, but the definitive diagnosis can only be determined post-mortem, or after death. Only then can we actually look into the brain for specific proteins that characterize the type of dementia. In the table below, the most common types of dementia and their general characteristics are summarized.
| Dementia | Clinical symptoms | Brain regions | Genes (most common) | Proteins/neuropathology |
| Alzheimer’s Disease (AD)1–4 | Progressive memory loss, impaired executive functioning, disorientation | Frontal, parietal, temporal, occipital lobe, hippocampus, amygdala, midbrain | APOE* (sporadic) PSEN1, PSEN2, APP (familial) | Amyloid-β (Phosphorylated) tau Neurofibrillary tangles |
| Frontotemporal Dementia (FTD)1,5,6 | Social cognition, language, movement, personality & behavioural changes | Frontale cortex, temporale cortex, cingulate cortex | C9orf72, GRN MAPT | TDP-43 Tau Fus |
| Dementia with Lewy bodies (DLB)1,7–9 | Visual hallucinations, mood changes, attention & alertness fluctuations, parkinsonism | Hersenstam, middenhersenen, sensorische gebieden, motorische gebieden | SNCA LRP10 | α-Syn Lewy lichaampjes |
| Vascular Dementia (VaD)10 | Cognitive impairment as a result of vascular damage, attention deficit, executive function, memory, language | Fronto-striatale circuits, hippocampus | APOE MTHFR | Atherosclerose in hersenvaten, infarcten |
Forgetting where you’ve put your keys, if you’ve turned off the stove, or can’t think of the word that’s on the tip of your tongue: is this normal, or are these symptoms of dementia? When do you start worrying? When do you go to the doctor? Didn’t my neighbour get dementia at 63? These are all questions that older adults start thinking about around or after their midlife crisis, either questions they ask about themselves or someone in their environment. Usually, it takes a few months, even years, before symptoms become progressively worse and people decide that it’s time to visit your doctor. Your GP may do several tests, such as a Mini-mental state examination (MMSE), which is a questionnaire that highlights memory, language, and concentration. The score of your MMSE provides a (rough) indication of your cognitive abilities; the higher your MMSE score, the better your cognitive abilities. Of course, it’s not that black and white, as age and education are taken into account during this test. When your GP isn’t sure about your situation, or has an indication that further testing is needed, you can get a referral to a specialist at a memory clinic or the neurology department at a hospital. A specialist can do more extensive testing, such as more cognitive tests, biomarker testing in blood or cerebrospinal fluid (CSF), imaging testing, or even genetic testing if there is an indication that the type of dementia could be hereditary.
So... what now? Why is there no treatment for dementia yet? How do these treatments develop? Within the field of drug development, the major focus is on AD. This is mainly because there are actual protein biomarkers in blood serum and in CSF, such as Aβ and tau/pTau levels that can indicate AD pretty well. Aβ proteins are present in a healthy brain, but in AD they form amyloid plaques outside of our brain cells. Tau proteins are present in neurons, but in AD and other dementias, tau proteins can also clump together and form neurofibrillary tangles inside our neurons. Together, these protein clumps of Aβ and tau disturb healthy connections between our brain cells, resulting in our brain tissue dying off. These biomarkers are not available (yet) for the other types of dementia. To make it even more complicated, the actual diagnosis of a type of dementia and subtype is based on neuropathology. These are markers, such as proteins or vascular damage, that we can only study in the brain tissue itself. As mentioned before, this can only be done after death, as it’s not really an option to dig into our brains to look for these proteins. This means that a diagnosis is only based on clinical symptoms and some biomarkers, if you’re ‘lucky’. This complicates the process a bit, because with all these different types and subtypes of dementia, and variable symptoms, how can we pinpoint the mechanisms that we want to tackle with treatment? And these proteins (Aβ and tau) also have a function in a healthy brain, right? This adds to the complexity of dementia research, where points such as the following may arise:
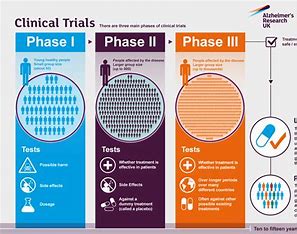
In the case of AD, most drug developments are aimed at Aβ clearance from the brain. Among neuroscientists, clinicians, and neuropsychologists, there are a lot of controversies and discussions ongoing on what aspect of AD we should focus on, and what implications this has on the quality of life of the patient. Current therapeutic drugs are so-called monoclonal antibodies, which are antibodies that bind to one target specifically. When bound to the target, in this case to Aβ, the antibody can induce a specific cell signal, such as the activation of the immune system, whereafter the bound Aβ is cleared from the system11,12. These types of treatments are tested in clinical trials, which are studies that test the efficacy and safety in terms of the effects on human health outcomes. To give you a realistic timeframe, it may take up to 10 to 20 years to get from start (mechanism discovery) to finish (approved treatment). According to the World Health Organization (WHO), there are 4 phases of biomedical clinical trials:
In the last couple of years, there has been some rotation in therapeutic interventions in the AD field, where the current or most recent drugs Aducanumab (Biogen) and Lecanemab (Eisai/Biogen) got accelerated approval by the U.S. Food and Drug Administration (FDA) after phase III clinical trials. This is where the controversies start, and where we have to dig into the data and trials a little bit. First of all, these studies were done in a randomized, double-blind, and placebo-controlled manner13 for 18 months, where participants were treated via injections every couple of weeks with different dosages of the treatment. Afterward, the participants were tested for cognition, as well as Aβ and tau/pTau levels, and the occurrence and severity of side effects. The data from these clinical trials show significant improvements in Aβ levels, some improvements in tau/pTau levels, and significantly less cognitive decline compared to placebo groups. This sounds pretty good, but there were some critical points here. First of all, the most improvements were seen in higher doses of the above-mentioned drugs, and it can be perceived as rather invasive to get this treatment injected regularly. Moreover, it's important to note that the participants had early AD, which includes mild cognitive impairment to mild AD. Lastly, we have to discuss the side effects; in the studies, they speak of amyloid-related imaging abnormalities (ARIA). These abnormalities present themselves as swelling in the brain or small spots of bleeding in/on the surface of the brain, possibly resulting in symptoms such as headache, nausea, and even seizures or brain shrinkage14,15. Moreover, these clinical trials were over 18 months, meaning that there isn’t much known about the long-term effects of these treatments beyond that period.
Based on inconsistent or ‘contrasting’ results, Aducanumab’s approval was withdrawn11,15, but Lecanemab has been administered at several U.S.-based clinics in recent times. Can we expect EU approval for Lecanemab soon, and what does this mean? If we take a critical look at the data and implications on care and quality of life, is Aβ clearance enough?16 Is the decrease in cognitive decline enough to improve quality of life in the long run? Isn’t it unfair that this may only be an option for early AD, and will not have any meaning to all those people whose AD is more severe? Is it worth risking bleeding in the brain, and is it worth the costs (we can assume that these treatments will not be cheap)? On the other side, we can also look at it from a different perspective: side effects may be quite severe, but chemotherapy in cancer treatment also comes with a busload of severe side effects, and people will still go for it. With AD treatment, it’s the same principle: a specialist can offer a treatment, and it’s up to the patient to decide on giving the green light for something like Lecanemab. For now, that’s the most we can do, and we’ll continue doing more research on this. Fortunately, with current ongoing research, we are learning a lot about the mechanisms of dementia, and innovative technologies are developing rapidly. So even though we don’t have a lot to hold on to right now, we have to remember that we have already made incredible steps in diagnostic procedures and therapeutic prospects. Dementia researchers are very hopeful that we will be able to cure dementia one day, so we’ll be too!
Author: Lotte Smit
1. Kovacs GG. Molecular pathology of neurodegenerative diseases: Principles and practice. J Clin Pathol. 2019;72(11):725-735. doi:10.1136/jclinpath-2019-205952
2. Montine TJ, Phelps CH, Beach TG, et al. National institute on aging-Alzheimer’s association guidelines for the neuropathologic assessment of Alzheimer’s disease: A practical approach. Acta Neuropathol. 2012;123(1):1-11. doi:10.1007/s00401-011-0910-3
3. Teunissen CE, Verberk IMW, Thijssen EH, et al. Blood-based biomarkers for Alzheimer’s disease: towards clinical implementation. Lancet Neurol. 2022;21(1):66-77. doi:10.1016/S1474-4422(21)00361-6
4. Kennisinstituut van de Federatie van Medisch Specialisten. Dementie: Richtlijn. Published online 2021.
5. Teunissen CE, Elias N, Koel-Simmelink MJA, et al. Novel diagnostic cerebrospinal fluid biomarkers for pathologic subtypes of frontotemporal dementia identified by proteomics. Alzheimer’s and Dementia: Diagnosis, Assessment and Disease Monitoring. 2016;2:86-94. doi:10.1016/j.dadm.2015.12.004
6. Pijnenburg YAL, Verwey NA, van der Flier WM, Scheltens P, Teunissen CE. Discriminative and prognostic potential of cerebrospinal fluid phosphoTau/tau ratio and neurofilaments for frontotemporal dementia subtypes. Alzheimer’s and Dementia: Diagnosis, Assessment and Disease Monitoring. 2015;1(4):505-512. doi:10.1016/j.dadm.2015.11.001
7. Gomperts SN. Lewy body dementias: Dementia with lewy bodies and Parkinson disease dementia. CONTINUUM Lifelong Learning in Neurology. 2016;22(2, Dementia):435-463. doi:10.1212/CON.0000000000000309
8. Quadri M, Mandemakers W, Grochowska MM, et al. LRP10 genetic variants in familial Parkinson’s disease and dementia with Lewy bodies: a genome-wide linkage and sequencing study. Lancet Neurol. 2018;17(7):597-608. doi:10.1016/S1474-4422(18)30179-0
9. Parnetti L, Tiraboschi P, Lanari A, et al. Cerebrospinal Fluid Biomarkers in Parkinson’s Disease with Dementia and Dementia with Lewy Bodies. Biol Psychiatry. 2008;64(10):850-855. doi:10.1016/j.biopsych.2008.02.016
10. O’brien JT, Thomas A. Series Non-Alzheimer’s dementia 3 Vascular dementia. www.thelancet.com. 2015;386. Accessed April 30, 2024. www.thelancet.com
11. Söderberg L, Johannesson M, Nygren P, et al. Lecanemab, Aducanumab, and Gantenerumab — Binding Profiles to Different Forms of Amyloid-Beta Might Explain Efficacy and Side Effects in Clinical Trials for Alzheimer’s Disease. Neurotherapeutics. 2023;20(1):195-206. doi:10.1007/S13311-022-01308-6
12. van Bokhoven P, de Wilde A, Vermunt L, et al. The Alzheimer’s disease drug development landscape. Alzheimers Res Ther. 2021;13(1). doi:10.1186/s13195-021-00927-z
13. Hariton E, Locascio JJ. Randomised controlled trials-the gold standard for effectiveness research HHS Public Access. BJOG. 2018;125(13):1716. doi:10.1111/1471-0528.15199
14. van Dyck CH, Swanson CJ, Aisen P, et al. Lecanemab in Early Alzheimer’s Disease. N Engl J Med. 2023;388(1):9-21. doi:10.1056/nejmoa2212948
15. Knopman DS, Jones DT, Greicius MD. Failure to demonstrate efficacy of aducanumab: An analysis of the EMERGE and ENGAGE trials as reported by Biogen, December 2019. Alzheimer’s & Dementia. 2021;17(4):696-701. doi:10.1002/ALZ.12213
16. Liu KY, Villain N, Ayton S, et al. Key questions for the evaluation of anti-amyloid immunotherapies for Alzheimer’s disease. Brain Commun. 2023;5(3). doi:10.1093/BRAINCOMMS/FCAD175